In the rapidly evolving landscape of wearable technology, ensuring compliance with regulatory standards such as the FDA (Food and Drug Administration) and HIPAA (Health Insurance Portability and Accountability Act) is critical. Whether you’re developing fitness trackers, medical devices, or other health-related wearables, meeting these stringent requirements is essential for market approval and the secure handling of protected health information (PHI).
In this article, we’ll go through the process of preparing submissions for both FDA and HIPAA. Let’s get started!
Understanding FDA Compliance
The FDA approval process is meticulously designed to ensure that medical devices are both safe and effective for public use. It is essential for developers and manufacturers to understand the key aspects of this process to navigate it successfully. According to the FDA, approximately 76% of medical devices receive approval on their first submission when proper compliance procedures are followed. This high success rate underscores the importance of adhering to established guidelines and protocols.
One critical element of the FDA approval process is documentation. Developers must provide comprehensive documentation that covers the entire development process, including testing results and traceability matrices. This thorough documentation is vital for demonstrating that the device meets all necessary safety and efficacy standards.
Furthermore, the wearable medical device market is experiencing significant growth. According to Grand View Research, the global market is expected to reach $87.5 billion by 2027, growing at a compound annual growth rate (CAGR) of 27.9% from 2020 to 2027. This rapid expansion highlights the increasing demand for compliant and innovative medical devices, making it even more crucial for developers to achieve FDA approval.
Key Steps in the FDA Approval Process
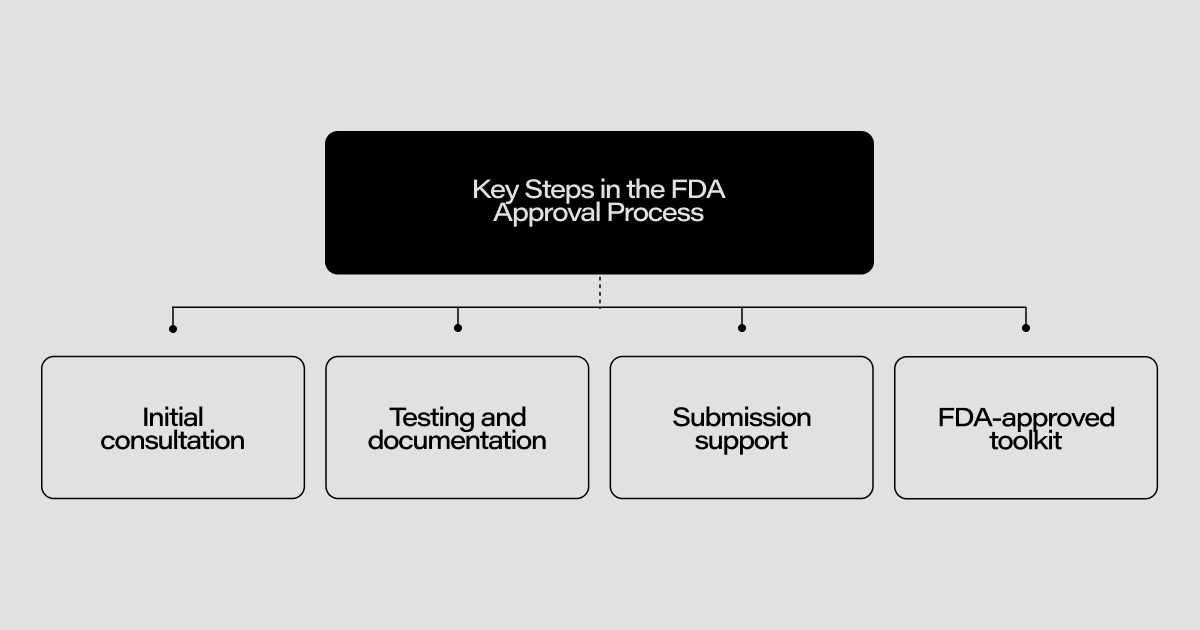
The initial step in the FDA approval process involves an initial consultation where developers must thoroughly understand the device and outline specific FDA requirements. This foundational phase sets the stage for the subsequent development phases. During the development stage, it is crucial to incorporate FDA guidelines into the design and development processes. This ensures that the device meets all necessary standards from the outset, thereby avoiding significant rework later.
Once the development phase is complete, the focus shifts to rigorous testing and documentation. Thorough testing is conducted to validate the device's safety and efficacy, and meticulous documentation of all data is essential. This comprehensive documentation serves as evidence that the device meets all regulatory requirements.
The final step involves submission support, where developers prepare and submit all the necessary documentation to the FDA. Proper submission ensures that the device is reviewed efficiently and has a higher chance of receiving approval on the first submission.
On top of that, this strict process applies also to the very toolkit one has to use. For example, you can’t use any spreadsheet tool; it needs to be approved by FDA. This additional safety measure makes the whole submission even more daunting.
Ensuring HIPAA Compliance
HIPAA compliance is crucial for protecting health information and ensuring user trust. Non-compliance can be costly, with potential fines averaging $6.5 million for healthcare organizations (Ponemon Institute).
Key HIPAA Requirements
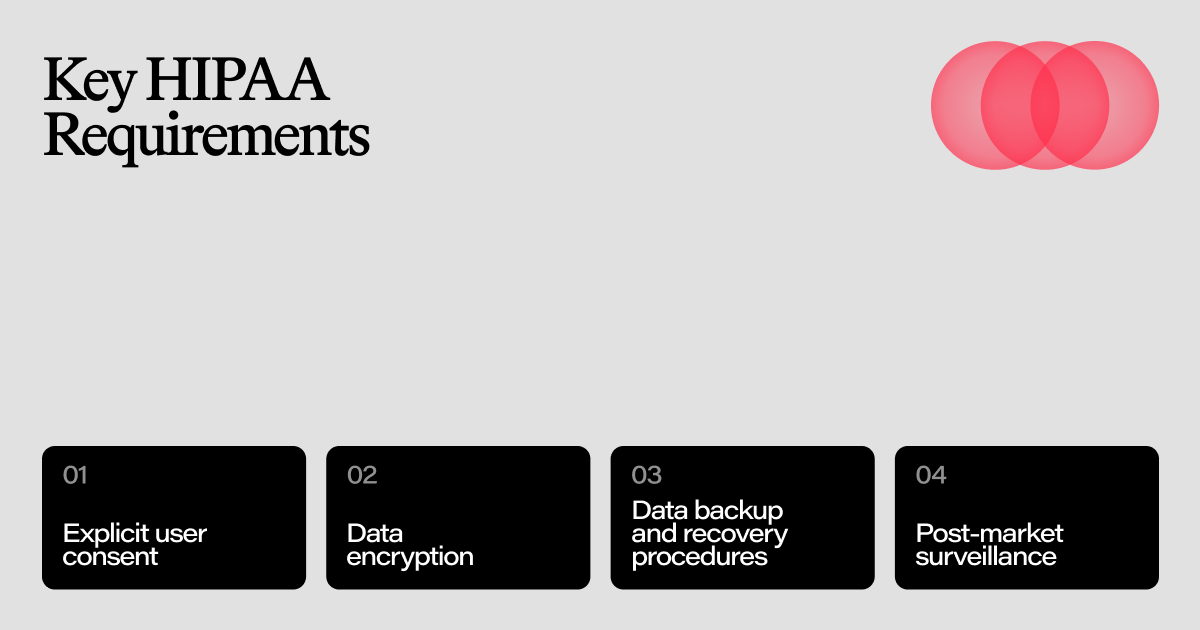
Ensuring HIPAA compliance is crucial for protecting health information and maintaining user trust. One of the key requirements is to obtain explicit user consent through a clear and transparent process. This involves informing users about how their protected health information (PHI) will be used and ensuring they agree to these terms. Additionally, a detailed privacy notice must be provided, explaining how PHI is handled and safeguarded.
Data encryption is another critical requirement. Implementing encryption protocols helps protect electronic PHI (ePHI) both at rest and in transit. Access control measures must also be in place to ensure that only authorized personnel can access ePHI, utilizing user authentication and role-based access control mechanisms. Maintaining detailed audit trails of all actions taken with ePHI is essential for accountability and regulatory compliance.
Furthermore, robust data backup and recovery procedures are necessary to ensure that ePHI is not lost due to unforeseen incidents. Secure communication channels must be used to transmit ePHI, ensuring that the data remains protected during transmission. Business Associate Agreements (BAAs) should be established with third-party service providers to ensure they also comply with HIPAA requirements.
Compliance monitoring and enforcement mechanisms should be implemented to conduct regular audits, security testing, and corrective actions as needed. Finally, post-market surveillance is crucial for monitoring the app's performance and addressing any safety or quality issues that may arise after the product has been launched. By adhering to these comprehensive measures, organizations can effectively ensure HIPAA compliance and protect user health information.
Best Practices for FDA and HIPAA Compliance
Achieving FDA and HIPAA compliance requires careful planning and execution. One of the best practices to keep in mind is to document everything. Maintaining detailed records of all development and testing activities is crucial. This comprehensive documentation serves as evidence of compliance and helps in tracking the progress and integrity of the project.
Another important practice is to integrate requirements early. Starting with FDA and HIPAA guidelines in mind from the outset can prevent major rework later. By incorporating these guidelines into the initial design and development processes, developers can ensure that the device meets all necessary standards right from the beginning.
Ensuring data traceability is also vital. Keeping track of all data sources and accurately mapping them to requirements is essential for compliance. This meticulous tracking helps in validating the data and ensuring that it aligns with regulatory expectations.
Regular reviews and audits are necessary to ensure ongoing compliance throughout the development process. Conducting these reviews helps in identifying any potential issues early and allows for timely corrective actions. This proactive approach ensures that the development process remains aligned with regulatory standards.

Collaborating closely with FDA and HIPAA consultants is another key practice. Working with experts who understand specific requirements and expectations can provide valuable insights and guidance. This collaboration ensures that the development process adheres to all necessary regulations and increases the likelihood of successful compliance.
Bug Bash for Bulletproofing the Software
The preparation for FDA submission provides a great opportunity to conduct a Bug Bash: an event where team members from various departments come together to identify and document bugs in an application. This event can take place before a major release or as part of a quick overview of upcoming features. The goal is to gather as much feedback as possible to provide to business partners and customers.
The preparation for a Bug Bash event begins with an organizer, who can be anyone from a project manager to a QA or developer, creating a document containing all the necessary information about the project. This document is typically based on a template. The organizer then surveys team members to determine their availability and plans the event with at least a week's notice. The QA responsible for the project prepares and sends this document, along with any necessary information, to the team as early as possible. Before the event, the QA sends all essential files, such as screenshots or test data, via email to the team.
During the event, the team gathers at the scheduled time. The organizer begins by explaining the objectives and process of the Bug Bash. Team members then spend about an hour identifying bugs and providing feedback. To make the event more engaging, a competition can be introduced where the person who finds the most bugs wins a prize.
After the event, the findings are reviewed to ensure all identified bugs are documented.
FAQs About FDA and HIPAA Compliance for Wearable Devices
What is FDA approval, and why is it important for wearable devices? FDA approval is a certification that ensures a device meets the necessary safety and efficacy standards. It’s crucial for market access, especially for health-related devices.
How long does the FDA approval process take? The timeline can vary depending on the device and its complexity. However, having a compliant software development process can significantly streamline the timeline.
What is HIPAA compliance, and why is it important? HIPAA compliance ensures the protection of health information and privacy. It’s essential for any device handling ePHI to ensure user trust and regulatory adherence.
Can both software and hardware aspects be addressed for FDA and HIPAA compliance? Yes, both aspects need to be addressed. While software development is crucial, seamless integration with hardware is also essential for compliance.
What kind of documentation is required for FDA approval and HIPAA compliance? Documentation includes detailed records of development processes, testing results, traceability matrices, privacy notices, user consents, and any other data that supports the device’s compliance with FDA and HIPAA standards.
Is ongoing support necessary after the submission? Yes, ongoing support is necessary to address any queries from the FDA and ensure continuous HIPAA compliance.
How Intent Can Help
At Intent, we are committed to helping you navigate the complex landscape of FDA and HIPAA compliance for your wearable devices. Here’s how we can assist:
FDA Compliance Consultation: Expert advice on meeting FDA standards.
HIPAA Compliance Consultation: Guidance on ensuring HIPAA compliance.
Software Development: Custom software solutions designed with FDA and HIPAA compliance in mind.
Quality Assurance: Rigorous testing to ensure software reliability, accuracy, and security.
Documentation Support: Assistance with preparing comprehensive documentation for FDA submission and HIPAA compliance.
Post-Submission and Compliance Support: Ongoing support to address any FDA or HIPAA queries and ensure smooth approval and compliance.
At Intent, our experience in creating software for IoT devices, particularly in the health and fitness sectors, positions us uniquely to help you achieve both FDA and HIPAA compliance. We provide end-to-end support, from development to documentation, ensuring your software meets all necessary standards.
Need help with your wearable device project? Contact us; we have ample experience with connected devices, including projects such as Oura.

Agnieszka Wach
Senior Quality Assurance Engineer & QA Team Lead

